-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Ali Ali1*, Wissam Zam and Razan Hasan
Corresponding Author: Ali Ali, Department of Food Technology, Faculty of Technical Engineering, Tartous University, Tartous, Syria
Received: May 21, 2022 ; Revised: July 05, 2022 ; Accepted: July 08, 2022 ; Available Online: Sep 08, 2022
Citation: Ali A, Zam W & Hasan R. (2022) Study of the Mechanical and Antibacterial Properties of an Edible Polymeric Film of Salep Modified with Pistacia Leaves Extract. J Stem Cell Ther Res, 1(1): 1-9.
Copyrights: ©2022 Ali A, Zam W & Hasan R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
Background: Due to the growing demand for microbiological safe foods, the ability of edible films to extend the shelf life of food, as well as their important role as a carrier of a wide range of food additives such as antioxidants and antimicrobials.
The objective of the present study was to investigate the effect of the extract of Pistacia leaves on the mechanical and antibacterial activity properties of edible salep film fortified with this extract. The extract was added with three different concentrations 1, 3, and 5% (w/w).
The modified salep films with three different concentrations of pistacia leaves extract had antibacterial activity. Both concentrations 3% (w/w) and 5% (w/w) of newly formed salep film, achieved the same antibacterial activity compared to newly formed control film. The storage of the control film and the films fortified with pistacia leaves extract for a period of one and two months led to an increase in bacterial load, compared to the bacterial load in newly formed films. A significant decrease in moisture content by 16.66% compared to control film, also a decrease in (pH) by 26.19% compared to control film when extract was added at a concentration of 5% (w/w). 5% (w/w) of extract had the greatest effect on improving the tensile strength TS by 83.32% (w/w). A significant decrease in Eb% was found by 17.87% with the increasing concentration of leaves extract from 0% (w/w) to 5% (w/w).
Keywords: Salep film, Pistacia leaves extract, Polyphenolic compounds, Antibacteria compounds, Mechanical properties
INTRODUCTION
Edible films have an increasing attention during past few years as biodegradable addition to its role by reducing environmental pollution caused by using traditional plastic films [1]. An ideal edible film contains three main ingredients; film forming material, additives, and the appropriate solvent, so this depends on the natural solubility properties of the film materials. Also; proteins, polysaccharides and fats are considered the most important components of the films [2]. Salep is a polysaccharide; it can be used to form biopolymer edible films for packaging food products with low and medium water activity [3]. Many trends have also emerged to reinforce edible films with natural, healthy-safe antimicrobial (GRAS) instead of chemical antimicrobials that endanger human health [4,5]. Diffusion of antimicrobials from the film depends on size, polarity, shape of the diffusing molecule, in addition to the chemical composition of the film and the degree of molecular crosslinking [6]. Phenolic compounds are distributed through the plant kingdom, and many chemical studies have proved that the Pistacia plant has secondary metabolites, and these contain various species of bioactive phenolic compounds [7]. Numerous studies have also demonstrated the importance of these compounds as antioxidants and antimicrobials [8,9]. Pistacia terebinthus extracts showed an antibacterial activity against Staphylococcus aureus, and an inhibition of glucuronidase enzyme (GUS) activity of bacteria Escherichia coli by ratio 92.4%. methanaolic extracts obtained from the leaves of Pistacia terebinthus were observed to be rich in phenolic compounds. Kaya were recorded that chitosan film incorporated with leaves extract gave the highest efficacy antimicrobial activity against negative gram bacteria as, P. microbilis, P.vulgaris, P. aeruginosa, E. coli compared to the control.
In a study conducted by Ekrami [3], it was stated that salep mucilage films enriched with pennyroyal extract had an antibacterial activity against Yersinia enterocolitica, Staphylococcus aureus, and Shewanella putrefaciens with increasing concentration from 0.5 to 1.5% (v/v), while the control had no significant influence.
Moisture content is a limiting factor for the biodegradability of films, and water sensitivity is considered one of the main problems facing polysaccharides films [10]. As well as, good mechanical properties are among the basic requirements that must be distinguished by edible packaging. The poor elasticity and tensile resistance of the films leads to cracking during production, handling or storage [11].However, the formation of edible salep film and reinforce it with pistacia leaves extract has never been investigated before. Therefore, it seems notable to include this study within this research. The purpose of this study is to evaluate the effectiveness of different concentrations of this extract on mechanical and bacterial properties of the film.
MATERIALS AND METHODS
Chemicals
All the solvents and reagents were of the highest purity required for each application. Extra pure acetone was obtained from Sharlau (Spain), extra pure glycerin was obtained from Eurolab (UK), Plate Count Agar; TGE-Agar was supplied by Sifin (Germany), and Salep powder was purchased from Kahramanmaras (Turkey) peptone water.
Preparation of Pistacia leaves extract
Pistacia leaves have been collected from the countryside of Tartous city (Syria) at the beginning of August. The crude powdered leaves were extracted by soaking 1 g of the powder in 50 ml of 40% (w/v) acetone at 40°C for 15 min [7].
Preparation of salep films
The content of salep of all the film-forming solutions was 1% (w/w). The salep solution was prepared by dissolving 1 g of salep powder in 100 g of distilled water in 45°C water bath with mechanical stirring until completely dissolved, and 1.3 % (w/w) glycerin was dissolved in salep solution. After mixing, the solution had been incorporated with different concentration of pistasia leaves extract (0, 1, 3, 5 % w/w). The mixtures were homogenized for about 20 min using a mechanical mixer (RZR 2021, Heidolph, Germany), at a constant speed of 300 (rpm) and 45°C. Next, 100 mL of solution was poured slowly onto the center of clean, pre-sterilized glass plate by solvent casting method. The plates were set horizontally using a mercury ruler for uniformity the thicknesses of films. Then, the solutions were left to set at room temperature 25°C for 24 h until the bubbles were cleared.
After that, the solutions were dried at 45°C in a laboratory (oven FN 500, nǜve, Turkey) for 36 h. The films were peeled from the plates and stored in the desiccator at 25±1°C and relative humidity at 52% for two days prior to analysis according to ASTM D618-61. The steps of preparation films was reported by (Djomeh) and (Ekrami) with little modifications [3].
The percentage of leaves extract was coded with the symbols PLE (Pistacia Leaves Extract) (0, 1, 3, 5% w/w) and they are in order (control, 1% extract, 3% extract, 5% extract).
Films pH
1 g of each film was dissolved in 25 [g] of distilled water and was left until completely dissolved, then pH of each solution was measured using a pH meter (EZDO, Taiwan) at 25±1°C.
Moisture content
Moisture content (MC %) was determined by drying films at 105°C until constant weight. The weight loss of the sample was measured [12]. Three replicates of each sample were tested and the results were expressed as a percentage and MC% was calculated using the following equation (1) [13]:
Where Mi is the Weight of the plate with the film piece before drying, Mp is the Weight of the plate, and Md is the Weight of the plate with the film piece after drying.
Bacterial analysis of films
The test was carried out in the microbial laboratory of Tartous port, and the procedures were applied according to the Syrian Standard Specification for the General Census of Microorganisms (MSS No. 600 of 2005).
18.5 g of Plate Count Agar (PCA) was added to one liter of distilled water, and then was boiled until completely dissolved. It was placed in an autoclave (Astell) at 121°C for 15 min, left to cool until to turn into a solid state and then placed in a microwave (wattar) at 40°C for 5 min to turn into a liquid state.
The initial suspension of the film and the appropriate decimal extensions were prepared according to Syrian Standard No. (2228). The film was digested by blending it with peptone water in a ratio (1:10), and macerated in a stomacher (BagciMixer, Interscience) within less than 0.5 [min] according to the calibration of the device.
Near flame, 1ml of each dilution was added by sterile pipettes into separate, duplicate, appropriately marked Petri plates. 15 ml of plate count agar (cooled to 44-47°C) was added to each plate within 15 min of original dilution. Immediately sample dilutions were mixed with agar medium thoroughly and carefully.
Then, agar was allowed to solidify on a cool horizontal surface. Petri plates were inverted and incubated by an incubator (Bechickung100-800) at 37±1°C for 48 h.
After incubation, the enumeration of bacterial colonies was performed using a colony counter (glexy230).The number of microorganisms per 1 ml or 1 g of the sample was calculated using the following equation (2):
(2)where (TCG) Total Count of Germs: is The number of microorganisms per 1[ml] or 1[g] of product [CFU/g].
C: is the total number of colonies in the plate.
d: is the coefficient of plate dilution in, the bacterial colonies appeared.
The enumeration of bacterial colonies was studied on different four newly formed salep films and after 30 and 60 days of storage.
Mechanical properties-Tensile Strength (TS) and Elongation at Break (Eb):
Tensile strength (TS) and percent elongation at break (Eb%) were determined according to the method ASTM D-882 (ASTM, 2001) by using a Universal Testing Instrument Model AI-5000 (Gotech, Taiwan) equipped with a 50 N static load cell.
Statistical analysis
Data were determined using the analysis of variance (ANOVA) method. Significant differences of treatment means were compared using the least significant difference (LSD) test at 5% significance level using (COSTAT, v.6.4) program.
RESULTS AND DISCUSSION
Films pH
Hydrogen number is a measure of acidity and alkalinity; it is also an important point of the relative quantity of free hydrogen and hydroxyl ions in a liquid.
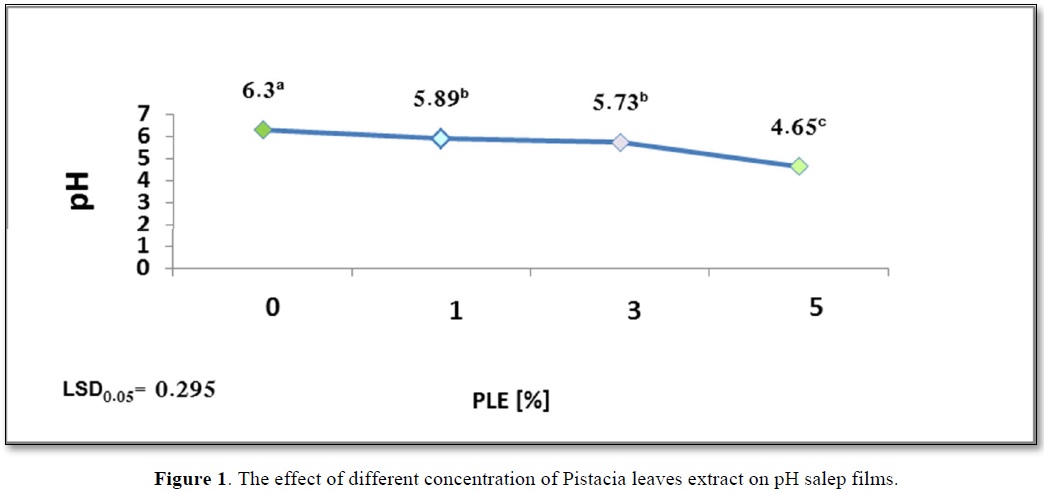
From the obtained results presented in Figure 1, it can be seen that acidic pH of salep film solution decreased with the increase of the PLE concentration. There was no significant difference in acidic pH5.89b and pH5.73b values for salep films containing PLE1% and PLE3% (w/per dry weight of salep) respectively.
The lowest pH4.65c value was found for the salep film with PLE5% (w/w). This is due to the fact that phenolic compounds have an acidic character and is considered one of the main components in pistacia leaves extract [14]. It is also noted from Figure 1 an increase of the film solution acidity with the increase in the concentration of phenolic compounds. Figure 1 shows the effect of different concentration of Pistacia leaves extract on pH salep films.
Moisture content
Moisture content of films depends on the chemical structure of the materials included and hygroscopic properties, and because of the high moisture content of salep-glucomanan, the crosslinking between biopolymer molecules is significantly declined in swelling structure of film. On the other hand, the high humidity of these films is due to the effect of water as a plasticizer [15].
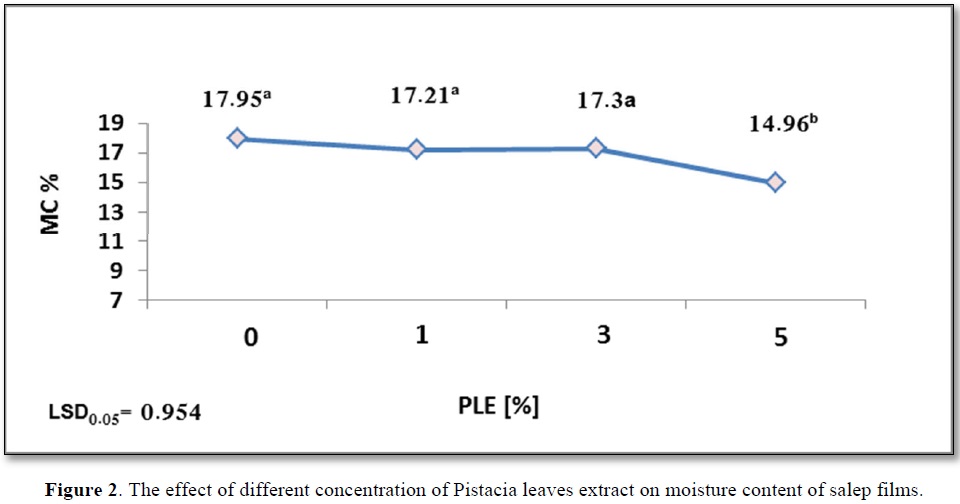
The effect of added PLE percentage to salep films on moisture level was evaluated in our study. According to the results shown in Figure 2, the moisture content greatly reduced from 17.95a to 14.96b with the increase in concentration of PLE from 0% to 5%. The lowest moisture content was measured for PLE5%. Whereas changes in PLE concentration from 0 to 3% had no significant influence on Moisture content of film, which recorded the following values 17.95a, 17.21a, and 17.3a.
However, this was explained by Kaya [16], that antioxidant phenolic compounds control moisture content of films. Hydrophilic groups, such as hydroxyl and carboxyl groups of pistacia leaves extract polyphenols, bind with water [16]. Our results are in accordance with those obtained by Norajit [17] who studied the effect of giseng extract including polyphenols on moisture content of alginate films. According to another study conducted by Ekrami [18], the possibility of binding between extract and hydrophilic hydroxyl groups of polymer increases [18]. Additionally, the decrease in film moisture content is due to the bonds formed between (glycerol-starch), which declines the absorption of water from the atmosphere. A decrease in moisture content is also due to organic acids that form crosslinking with starch, organic acids increase hydrophobic nature of surface and surface energy due to the formation of hydrogen bond [19]. Figure 2 shows the effect of different concentration of Pistacia leaves extract on moisture content of salep films.
Bacterial analysis of films
Many researchers have previously tended towards enhancing films and coverings solution with edible materials in order to improve physical and chemical properties of food and also to addition bioactive compounds, thus contributing with enhancement of the antioxidant and antimicrobial properties and in order to addition nutritional value to the film. Caririllo & Santiesteban-Lopez each visible colony is the result of germination of a single cell on agar surface. Table 1 shows the effect of storage time and concentration of pistacia leaves extract on the bacterial load [CFU/ml] of the different mixtures of studied salep films.

Uppercase for comparison within a single column-lowercase for comparison within a single row
The antibacterial activity of the edible Salep films has been given in Table 1.
Data presented in Table 1 show a decrease in total bacterial count as the concentration of extract increased from 0% to 5% w/w. The number of bacterial colonies of newly formed films varied from 35 x 102 CFU/ml to 1x102 CFU/ml, respectively. Moreover the total bacterial count was observed to growing significantly after 30 days of formation from 2 x 102 CFU/ml to 4 x 105 CFU/ml, respectively. The values also increased highly from 3 x 102 CFU/ml to 1x 106 CFU/ml, when the extraction concentration increased from 0% to 5% w/w, respectively after 60 days of storage.
No inhibitory activity of bacterial growth was observed for salep control film compared to another fortified with the extract. It was also found that salep film with PLE1% recently made had an inhibitory efficacy by 77% against bacterial colonies growth, by 94.3% for PLE3% incorporated into salep film and by 97.2% for salep film modificated by PLE5% compared to control film.
These results are constant with those showed by Xu [20], who found that konjac glucomannan (KG) did not have any Inhibitory effect on Staphylococcus aureus. The same results were also obtained by Li [21] on glucomannan film which had no antibacterial activity against E. coli, S. aureus, L. monocytogenes, B. cereus. Lu [22] also indicated that KG had no antibacterial activity against S. aureus, B. subtilis, E. coli, P. aeruginosa, Saccharomyces.
In order to investigate the effect of storage period on bacterial growth. It was noticed that the inhibition level of salep films decreased throughout storage period.
PLE5% had the best effectiveness of inhibition bacterial growth, as there was no significant difference between 200A and 300A CFU/ml after 30 and 60 days of storage, respectively. Otherwise, Salep film with PLE1% did not show a microbiological stability that mimics the microbiological stability observed at PLE3% and PLE5%.
As shown in Table 1, pistacia leaves extract had a significant antibacterial activity. This owing to containing volatile aromatic compounds, most important of which are polyphenols and flavonoids [16]. Kaya [16] even found that Pistacia terebinthus extract incorporated into chitosan film was more effectiveness against P. microbilis, P. vulgaris, P. aeruginosa, E. coli compared to control film.
These results are constant with those showed by Ekrami [18], on Salep mucilage film incorporated with Pennyroyal. Maryam [23] also revealed to similar results on agarcellulose bionanocomposite films functionalized with savory essential oil.
This owing to the decrease in the concentration of antimicrobial compounds as storage time increased. Our results are in accordance with those obtained by Gonzalez [24], who studied the effect of storage time on antimicrobial activity of chitosan–tea tree essential oil composite films.
Furthermore, the amount of remaining phenolic compounds of salep film during storage period are influenced by condition and used drying method, type, size, chemical and physical state of added phenols and antimicrobial agents [25].
The study also investigated the effect of film solution pH on its antibacterial activity. As reported in Figure 1, an increase in the acidity of edible film solution (low pH) was associated with an increase in the concentration of additive extract. Lower pH4.65c showing greater antibacterial activity than pH 5.89b and pH6.3a.
The same results were also conducted by Doughari, the values of acidity pH of tamarindus indica leaves extract from pH2 to pH6 improved the antibacterial activity of the extract whereas, a decline in antibacterial activity was obtained at alkalinity pH [26]. According to another study conducted by Maria, a positive antibacterial effect of apple puree-alginate edible coating enriched with lemon oil and oregano oil was observed at pH<3.6 compared to pH4.5 Grau [27] and Duan [28] on chitosan-lysozyme Film.
Glycerin raises the solubility and regular dispersion factor of the extract within film mold, thus, the effectiveness of the extract as an antibacterial agent increases [29].
Finally, the general bacterial census of bacteria should be not exceeding 1x 103 CFU/ml according to Witthuhn 2005.
Mechanical properties
Determination the mechanical properties of films is useful for knowing their applications in nutrient field, in some cases a high tensile strength is required to protect the food structure [30]. Mechanical properties reflect the films capacity to maintain the physical integrity of foods.
Several studies have shown the importance of formed crosslinks using crosslinking agents, by improving the mechanical properties and water-resistance property of films, as a result of formulation of internal bonds between polymer chains molecules [31]. It is very important to study the biological effects of crosslinking agents. Natural crosslinking agents, including polyphenols, have been turned instead of synthetic due to their toxic effect [32].
The effect of incorporating PLE in different proportions on mechanical properties of salep films is presented in Figure 3.
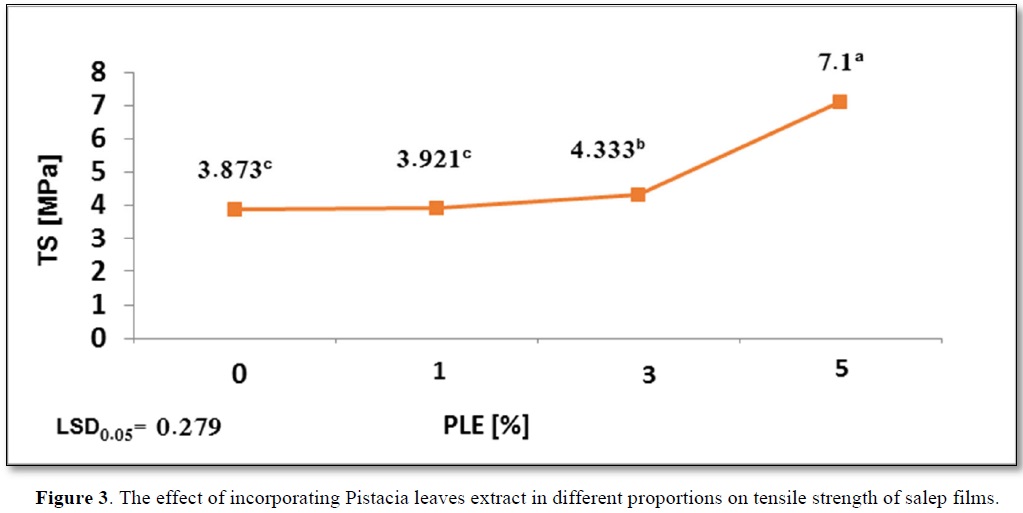
The relatively high Ts value of control film is due to the condensation of mannan chains, which leads to an increase in number of hydrogen bonds because of the decrease of acetyl group in glucomannan [15].
It was observed an increase in Ts to 7.1a Mpa at PLE5% as be noted in Figure 3. This is due to the effect of polyphenols compounds that bind to polymers such as polysaccharides by hydrogen bonds. The greater the number of hydroxyl groups, the greater the possibility of bonding with polysaccharides by hydrogen bonds [33].
Increasing the concentration of extract from 0% to 5% had a positive role in improving the tensile strength of salep film. Our results are in accordance with the results shared by Jaramillo [34], when fortifying starch film with yerba mate extract. Furthermore, the mechanical capabilities of salep is affected by the composition and concentration of the components and by changes in glycerin [3], and the added glycerin plays as a plasticizer without formulation covalent bonds with the biopolymer. Hydroxyl groups exist in glycerin constitute a hydrogen bond with the biopolymer molecules at hydroxyl and carbonyl sites as well as increase the steric volume and weakens the intermolecular forces [35].As reported in Figure 3, Ts salep film increased significantly at PLE3% and PLE5%, while there was no significant change in Ts at PLE1% compared to control film.
This is caused by the interact between polymer network and extraction polyphenols and the effect of polyphenols concentration, in addition to the variation in film structure and increasing its thickness according to increasing the concentration of extracted polyphenols. Du also revealed to similar results by utilizes different concentrations of apple skin polyphenols [36] as well as by application thyme extract on chitosan film [37].
Moreover, Li [38] explaining that these differences in the molecular composition of solution, which in turn effects on the molecular structure of film could be due to the difference in types of acids. Results in Figure 1 proved that solution acidity (pH) had a significant effect on the mechanical properties, which decreases as Ts increases. An increase in solution acidity (a decrease in pH) from pH 6.3a to pH 4.65c was observed with a significant increase in Ts from 3.873c to 7.1a. A similar trend was reported by Shivania and Ola [39], on starch film with added stearic acid, as also reported by Li [38], on edible film based on pullulan-chitosan.
This increase in TS values could be associated with formation of hydrogen bond between the acidic compound and starch molecules [40].
The effect of incorporating PLE in different proportions on tensile strength of salep films shows in Figure 3.
The salep incorporation in gradual higher concentration of PLE(1,3,5%) showed a significant equal reducing in Eb% = 170b%, as evident from Figure 4, compared to the control film which recorded the highest value of Eb% = 207a.
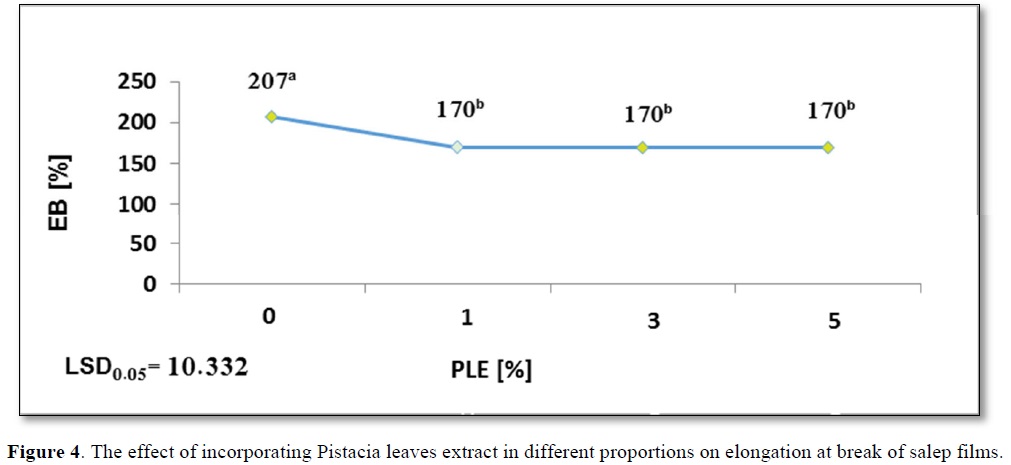
Chambi and Grosso [41] found that the high branched nature of glucomannan leads to a molecular movement and a weaker molecular matrix, which makes it free relatively. Subsequently, this is due to production more flexibility films.
The control salep film recorded MC% = 17.95a %, according to data presented in Figure 2, this owing to the hydrophilic nature of glucomannan [42]. Li [38] proved that the high moisture of film raises the molecular mobility, this in turn increases Eb% index. These results are contrary to those showed by Carlos [41], who found that no a direct relationship was reported between the humidity content and Eb% of film.
As previously mentioned, a reducing in moisture content of film containing PLE5% to 14.96b% and Eb% to 170b% compared to the control film. Lower water content reduces the movement capacity of film chain because water acts like a plasticizer [10].
In another study conducted by Bruce and Ubonrat [43], the same results were observed on chitosan film fortified with green tea extract. Addition of the extract leads to the development of structural discontinuities; these results are constant with those evaluated by Qin [44]. The effect of incorporating Pistacia leaves extract in different proportions on elongation at break of salep films shows in Figure 4.
CONCLUSION
The present study was conducted to optimize different percentages (1,3,5%) of extraction of pistacia leaves affecting tensile strength, elongation at break and antibacterial properties of polymeric salep film in order to improve these parameters. Results showed that all the concentrations had a significant effectiveness against bacterial growth. Both concentrations 3 and 5% achieved the superiority and the same efficacy. Microbes’ growth increased with the increasing time of film storage. The concentration 5% had the best effect on improving Ts. A significant decrease in Eb% was found with the addition of extracts. A significant decrease in moisture content and pH was found when extract was added at a concentration of 5%. Thus the concentration 5% was selected as the optimal extraction parameter. Salep film consists of salep 1%, glycerin 1.3% and PLE5% is suitable for packaging food products.
Despite the great developments in the field of health science and food production techniques, there has been a growing interest in the maintenance of general food quality. Based on the vital importance of leaves extract, it was invested by being adde to edible salep films. To our knowledge, this is the first study that adds the phenolic compounds of pistacia leaves extract to biopolymeric film based on salep. These films can be very suitable for being used in food packaging and it can be of great potential to raise the nutritional value of food. However, it is necessary to spot light on the importance of application the extraction of pistacia leaves to other polymers such as, sodium alginate, cellulose, starch, and flax seed gelatine in order to improve different parameters of films. Additional, modification of edible film with antimicrobials materials such as silver nanoparticles or zinc oxide particles and comparison with the antimicrobial efficacy of the manufactured salep films.
REFERENCES
No Files Found
Share Your Publication :